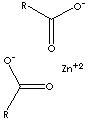|
ZINC STEARATE |
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 557-05-1 |
|
| EINECS NO. | 209-151-9 | |
| FORMULA | [CH3(CH2)16COO]2Zn | |
| MOL WT. | 632.33 |
|
| H.S. CODE | 2915.70.0150 | |
|
TOXICITY |
Oral rat LD50: 10 gm/Kg | |
| SYNONYMS | Stearic acid, zinc salt; Octadecanoic acid, zinc salt; | |
| Dermarone; Metallac; Talculin Z; Zinc distearate; Zinc octadecanoate; Zinco stearato (Italian); Zinkdistearat (German); Diestearato de cinc (Spanish); Distéarate de zinc (French); Other CAS RN: 8028-87-3, 72535-55-8 | ||
| PRICE |
U$3.50/kg CFR by sea for 1,000kgs |
|
|
SMILES |
[Zn+2].C(=O)([O-])CCCCCCCCCCCCCCCCC.C(CCCCCCCCCCC CCCCCC) (=O)[O-] | |
|
EXTRA NOTES |
EPA Pesticide Chemical Code 077002 | |
|
CLASSIFICATION |
Carboxylic Acid Salt |
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
various form of white solid without odor |
|
| MELTING POINT | 120 - 130 C |
|
| BOILING POINT | ||
| SPECIFIC GRAVITY | 1.095 | |
| SOLUBILITY IN WATER | Insoluble in cold water | |
| SOLVENT SOLUBILITY | Slightly soluble in benzene | |
| pH | 6.5 - 7.5 in slurry | |
| VAPOR DENSITY | ||
| log Pow | 14.44 (Octanol-water) | |
| OH RATE | 4.29E-11 (cm3/molecule-sec at 25 C Atmospheric) | |
| AUTOIGNITION |
790 C |
|
| NFPA RATINGS | Health: 0; Flammability: 1; Reactivity: 0 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT | 276 C | |
| STABILITY | Stable under ordinary conditions | |
|
EXTERNAL LINKS &GENERAL DESCRIPTION |
||
|
GENERAL DESCRIPTION: Zinc Stearate is used as a stabilizer for plastics with co-stabilizer of Ba-Cd soap. It is also used as a plasticizer in plastic industry as well as in cosmetics. It is used as a flatting and sanding agent in lacquers, coatings & inks. It is applied in tablet manufacturing. It is used as a drying lubricant and dusting agent for rubbers. It is used as a catalyst in chemical synthesis. It is used as a waterproofing additive in concrete, rockwool, textiles and paper. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
White Powder |
|
| Zn CONTENT |
12.5 - 14.0% |
|
|
MELTING POINT |
117 - 120 C |
|
| MOISTURE |
0.8% max |
|
| FREE FATTY ACIDS |
0.5% max |
|
| FINENESS |
99.0% (325 mesh) |
|
| TRANSPORTATION | ||
| PACKING | 25kgs, 1mt in bag in bag | |
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
GENERAL DESCRIPTION OF FATTY ACID |
||
| Fatty Acids are aliphatic carboxylic acid with varying length hydrocarbon chains at one end of the chain joined to terminal carboxyl (-COOH) group at the other end. The general formula is CnH2n+1COOH or R-(CH2)n-COOH. Fatty acids are predominantly unbranched and those with even numbers of carbon atoms between 12 and 22 carbons long react with glycerol to form lipids (fat-soluble components of living cells) in plants, animals, and microorganisms. Fatty acids all have common names respectively like lauric (C12), MyrIstic (C14), palmitic (C16), stearic (C18), oleic (C18, unsaturated), and linoleic (C18, polyunsaturated) acids. The saturated fatty acids have no solid bonds, while oleic acid is an unsaturated fatty acid has one solid bond (also described as olefinic) and polyunsaturated fatty acids like linolenic acid contain two or more solid bonds. Lauric acid (also called Dodecanoic acid) is the main acid in coconut oil (45 - 50 percent) and palm kernel oil (45 - 55 percent). Nutmeg butter is rich in myristic acid (also called Tetradecanoic acid ) which constitutes 60-75 percent of the fatty-acid content. Palmitic acid(also called Hexadecylic acid ) constitutes between 20 and 30 percent of most animal fats and is also an important constituent of most vegetable fats (35 - 45 percent of palm oil). Stearic acid ( also called Octadecanoic Acid) is nature's most common long-chain fatty acids, derived from animal and vegetable fats. It is widely used as a lubricant and as an additive in industrial preparations. It is used in the manufacture of metallic stearates, pharmaceuticals, soaps, cosmetics, and food packaging. It is also used as a softener, accelerator activator and dispersing agent in rubbers. Oleic acid (also called octadecenoic acid) is the most abundant of the unsaturated fatty acids in nature. | ||
GENERAL DESCRIPTION OF METALLIC SALTS OF FATTY ACIDS |
||
|
Metallic salts of fatty acids (called soap) are primarily used as cleansing
agent (mainly sodium- and potassium-) which their molecules attach readily to
both polar molecules (of water) and non-polar molecules (of grease or oil). The
long hydrocarbon chains are non-polar (and hydrophobic) repelled by water and
the salt end molecules are ionic (and hydrophilic) water soluble. Soaps differ
according to the type of fatty acid and length of the carbon chain and according
to the alkali employed. Fatty acids with longer chains are insoluble. If sodium
hydroxide is used as the alkali, hard soaps are formed; potassium hydroxide
yields soft soaps. Soap salts are used as insecticides, herbicides, fungicides
and algaecides. The lipophilic carbon chains infiltrate
and destroy the lipoprotein matrix of the insect's cell
membranes. Food grade soap salts are used also as general
purpose food additives. Aluminum, calcium, magnesium, lead, zinc or other metals are
used in place of sodium or potassium for soaps to be used in industry. Metallic
salts of fatty acids are used as stabilizer and plasticizer in plastic industry
as well as in cosmetics. They are used as flatting and sanding agents in
lacquers, coatings & inks. They can be applied in tablet manufacturing. They
are used as drying lubricants and dusting agents for rubbers. They are used as
catalysts in chemical synthesis and emulsifiers for emulsion polymerization of
synthetic rubber and resin which can be approved for use in food contact
applications. They are used as waterproofing additives and ointments.
|
||